Introduction
A Component Content Management System (CCMS) that implements the best-in-class DITA
standard for structured, component-based authoring can benefit organizations in many different
industries and sectors.
Whatever the industry, the core value proposition is built on:
- precision management and reuse of content in multiple publications
- the ability to track, search, filter and report on content using metadata (which is
especially critical in regulated industries) - mass collaboration to produce customer content at speed
- customized workflows to streamline authoring, review, approval and release of content
- the ability to branch and merge content with ease and accuracy, allowing authoring of
multiple document variants - management of content localization cycles for efficient multilingual publication
- ease of publication for content variants to any medium or delivery platform
A CCMS in Action
A CCMS adds value for virtually any industry by streamlining documentation processes,
creating efficiencies, improving content quality, and enhancing customer experience. Here are
some examples of a CCMS in action.
Learning and Training
In the learning and training space, content changes quickly. Content may vary depending, for
example, on jurisdiction, product variant, or role. Content is often supplied by Subject Matter
Experts but developed for distribution by training content developers or professional writers.
Content may be distributed online through a web portal, published to PDF, or pushed to an app
on a mobile device.
The right CCMS addresses all of these use cases with ease and efficiency. Component
authoring, metadata handling, filtering, and branch capabilities make production of content
variants fast and precise. SMEs can author, review, or update content through an intuitive web
interface while professional writers have access to a power authoring environment that provides
the capabilities they need. Content is easily published to any medium or look and feel.
Transportation
For transportation industries such as airlines, internal content must be rigorous and thorough
while satisfying detailed regulatory requirements for publication, change management, and
reporting. Such companies have exacting demands for policies and procedures as well as
training information. A CCMS offers the templating for precision authoring, sophisticated
publishing, granular metadata, and reporting capabilities needed to satisfy these demanding
requirements.
Automotive
Automotive documentation such as owner manuals and repair manuals have many
requirements, including the ability to reuse content for multiple product variants with mass
content reuse, adherence to regulatory compliance, parts information management (see
Manufacturing below), multilingual publication support, and integrated, intuitive SME review. In
addition, exacting publishing requirements may include owners’ manuals delivered directly to
the in-cabin screen on the vehicle. For these use cases, only a CCMS and component authoring
offer the power, versatility, configurability and publishing capabilities required.
Manufacturing
In manufacturing industries, the challenge includes both creation of technical documentation –
installation manuals, service manuals, and so on – and creation and management of Illustrated
Parts Catalogs (IPCs). In addition to the many strengths offered by a CCMS for technical
manuals, it is possible to integrate parts Bills of Materials (BOMs) from an Enterprise Resource
Planning (ERP) system and parts diagrams generated from CAD software directly into the
CCMS and to automate the generation of an IPC customized to the precise BOM configuration.
Parts in the manuals can link directly to the right part in the IPC.
The figure below shows a fully configured CCMS environment with parts capabilities using the
XDocs Manufacturing Suite and the XDelivery Documentation Portal.
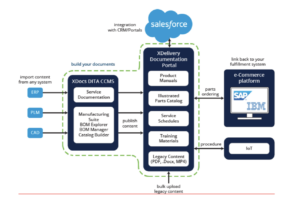
The Service Manuals and IPCs can be delivered to customers in a unified content delivery
portal with parts ordering integration, providing lower support costs, increased spare parts
revenue, and better customer experience.
Medical Technology Companies
A CCMS is essential to address the technical content challenges for enterprises in the heavily
regulated medical devices industry. Schema specializations and templates, coupled with the
powerful metadata modeling, harvest, search, and reporting capabilities of a CCMS, ensure that
content will meet customer and regulatory requirements. Flexible publication options mean that
content can be delivered online through a delivery portal, in PDF form, in a mobile app, or any
other format.
Medical technology companies tend to have global reach and need to serve markets in their
native languages. Some CCMSs provide localization management that allows you to rapidly run
localization cycles with Localization Service Providers (LSPs) and provide technical content for
global markets much faster and with far lower cost than localizing using traditional desktop
publishing tools.
Networking OEMs
A CCMS is a powerful tool for documentation authoring and delivery in technical organizations
such as networking Original Equipment Manufacturers (OEMs), particularly when it is optimized
for DITA. OEMs create complex networking switches and routers with sophisticated
management software. Installation and maintenance guides, network design guides, and
troubleshooting documents are typically required. Tight deadlines with requirements for
collaborative workflow and SME review are essential. The right CCMS has a configurable
workflow and intuitive web-based tools for SME review and contribution as well as power
authoring tools for technical writers. These features enable rapid development of accurate,
complete documentation.
In addition, many product variants require closely related documentation such as Command
Reference Guides. The reuse technologies of a CCMS provide a powerful solution. In particular,
the ability to branch and merge content at a granular level enables the authoring of separate
content streams for each release while filtering, reference from multiple maps, and other reuse
mechanisms allow document variants within individual releases. This ability to layer different
content reuse approaches is invaluable for such complex demands. All content can then be
published in virtually any media format.
It is also common practice for OEMs to rebadge their products for resellers. With a CCMS and
component authoring, rebadging at publish time can be fully automated.
How to Evaluate if a CCMS is Right for You
In many industries, organizations that produce technical user content can benefit from a CCMS.
But is a CCMS right for you? And if so, which one?
To answer this question, think about the specific needs of your company and your industry.
Consider the entire content lifecycle, your use cases, and your goals. How do you create,
update, review, approve, and release documentation? Does the current workflow satisfy your
needs and objectives? What does the ideal workflow look like?
Be careful not to try to replicate exactly what you do today. Some organizations are change-
averse, but the move to a CCMS opens up new possibilities to develop, review, update, and
manage content collaboratively with precision, rigor, efficiency, and speed, and to deliver
content in ways that improve customer experience. If a CCMS is right for you, with proper
analysis it should be possible to build a compelling business case.
Key areas where you should document your use cases and ideal state may include:
- Content reuse
- Workflow
- Authoring content
- Updating content
- Finding content
- Reviewing content
- Publication
- Content delivery
- Localization
- Metadata
- Searching and finding content
For each of these areas, how would a CCMS fit into your content processes? How would it
help? Would it provide efficiencies and save money? Would it improve quality? Simply help the
writing team to keep everything organized and moving forward? When coupled with a content
delivery platform, would it enhance the customer experience and drive additional revenue?
CCMS software can deliver value in all of these areas.
Once you have your use cases established, research and select a short list of CCMSs to
request vendor demonstrations. A CCMS is a large, feature-rich software system, so send your
use cases, information about your content processes and goals, and content samples to the
vendor. This will enable vendors to give you high quality demonstrations that address your
particular concerns so that you can determine if a CCMS is right for you.
Introducing the XDocs DITA CCMS
Bluestream’s XDocs DITA CCMS is a fully featured, modern CCMS that implements the DITA standard,
enabling technical documentation teams to manage all aspects of their content lifecycle. XDocs is
deployed in many industries and powers the documentation lifecycle for organizations scaling from lone-
writer startups to Fortune 500 companies with hundreds of authors.
With a power user interface integrated with the best-in-breed desktop Oxygen XML Author as well as an
intuitive, web-based editing interface for reviewers and content contributors, XDocs offers a superb blend
of power and ease of use.
The XDocs DITA CCMS excels in metadata management, enabling you to search, filter, query, and report
on your content based with precision. In addition, XDocs Release Management allows you to branch and
merge content for product variants, to create release snapshots, and so forth.
XDocs’ Localization Management manages localization cycles with ease and efficiency, allowing you to
send only changed content to your LSP while allowing authors to continue to modify source language
content. The system can be integrated with the LSP for automated content export and import.
XDocs is the only CCMS on the market that supports parts references for the manufacturing sector.
XDocs ingest Bills of Materials (BOMs) and makes part information referenceable in the documentation.
Furthermore the ability to ingest parts allows for both manual and automated production of Illustrated
Parts Catalogs (IPCs) in PDF and online formats.
XDocs DITA CCMS includes versatile publishing capabilities and can be paired with the tightly integrated
XDelivery Documentation Portal. XDelivery supports any document type including integrated IPCs that
can drive spare parts sales. XDocs coupled with XDelivery provides a better overall user experience
when interacting with documentation and reduces the burden on an organization’s Product Support team.
Whatever your industry or use case, it is likely that a CCMS would benefit your organization. Contact
Bluestream to learn more about the XDocs DITA CCMS.

